How to obtain nuclear energy. Educational program: How to obtain nuclear energy
At the end of the last century, scientists were surprised to discover that atoms, or rather the nuclei of atoms, spontaneously disintegrate into parts, emitting rays and heat. They called this phenomenon. And when they calculated it, they were even more surprised: 1 g of radium, if completely disintegrated, can provide the same amount of heat as 500 kg of coal when burned. But it is impossible to use this property - atoms decay so slowly that in 2000 years only half the heat is released.
It's like a big dam. The dam is closed, and the water flows in a small stream, from which there is no benefit.
Now, if the dam were opened, if people learned to destroy atoms!.. They would receive an endless ocean of energy. But how to do that?
They say that you can’t shoot a sparrow from a cannon, you need a small pellet. Where can you get a pellet to split the nucleus of an atom?
Scientists all over the Earth have been working hard for several decades. During this time, they learned how it works and found a “pellet” for it. It turned out to be one of the particles that is part of the nucleus - a neutron. It easily penetrates the atom and breaks the nucleus.
And then it turned out that the atoms of the metal uranium, having split, release new neutrons that destroy neighboring atoms. If you take a piece of uranium in which many nuclei will simultaneously decay and many new neutrons will be released, the fission process will grow like an avalanche in the mountains. An atomic bomb will explode.
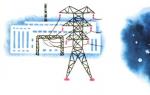
Diagram of a nuclear reactor. Thick black rods are neutron absorbers. The reactor heats the water, and then heats the water in the heat exchanger to a boil. The resulting steam rotates the turbine of the power plant.
Imagine that a large dam has collapsed. The water collected behind it will all rush down violently at once. The power of the flow is great, but it only causes harm, because it sweeps away everything in its path. It’s the same with an atom: the colossal energy of an explosion can only destroy. But people need nuclear energy to build. Now, if the atom gave up its reserves in such portions as we want! No energy needed - closed the damper. I needed - (How much do you need?) opened two or three doors: “Get what you asked for!”
And the man curbed the explosion.
Who is the main “worker” at the “nuclear plant”? Neutron. It is he who breaks the uranium nuclei. What if we remove some of the workers from the “factory”? Work will go slower.
This is exactly how a nuclear boiler, or nuclear reactor, works. This is a large well with thick concrete walls (they are needed to prevent radiation harmful to people from escaping outside). The well is filled with graphite, the same material from which pencil leads are made. There are holes in the graphite filling where uranium rods are placed. When there are enough of them, the required number of “working” neutrons appears and the atomic reaction begins.
To control it, in other holes there are metal rods that capture and absorb neutrons. These are the “gates” in the dam.
No energy is needed or there is a danger of explosion, the damper rods are instantly lowered, the neutrons emitted from the uranium nuclei are absorbed, stop working, and the reaction stops.

It is necessary for the reaction to proceed, the shutter rods are raised, “working” neutrons appear in the reactor again, and the temperature in the boiler rises (How much energy do you need? Get it!).
Nuclear reactors can be installed on nuclear power plants, on nuclear submarines, on a nuclear icebreaker. They, like ordinary steam boilers, will obediently turn water into steam, which will rotate the turbines. Five hundred kilograms of nuclear fuel - the contents of just ten suitcases - is enough for the Lenin icebreaker to sail all year round. Can you imagine how beneficial it is: you don’t need to carry hundreds of tons of fuel with you, you can take a more useful load instead; you can avoid going to the port to refuel for a whole year, especially since in the North this is not always easy to do. Yes, and stronger cars can be installed...
In existing nuclear reactors, energy is obtained by destroying nuclei consisting of a large number of particles (in uranium nuclei, for example, there are more than two hundred of them). And although there is still a lot of such fuel on Earth, someday it will run out... Is there a way to obtain nuclear energy from other substances? And scientists found it!
It turned out that atoms, which have only two particles in the nucleus: one proton and one neutron, can also serve as a source of energy. But they give it away not during fission, but during the connection, or, as they say, during fusion, of two nuclei.
To do this, hydrogen atoms need to be heated to many millions of degrees. At this temperature, their cores begin to move at tremendous speed and, having accelerated, can overcome the electrical repulsive forces that exist between them. When they get close enough, nuclear attractive forces begin to act and the nuclei merge. Thousands of times more heat is released than during nuclear fission.
This method of producing energy is called a thermonuclear reaction. These reactions rage in the depths of both distant stars and the nearby Sun, which gives us light and warmth. But on Earth they have so far manifested themselves in the form of a destructive explosion of a hydrogen bomb.
Now scientists are working to force hydrogen nuclei to combine gradually. And when we learn to control thermonuclear reactions, we will be able to take advantage of the limitless reserves of energy contained in water, which consists of hydrogen and whose reserves are inexhaustible.
| <-- | --> |
An atom consists of a nucleus surrounded by clouds of particles called electrons(see picture). The nuclei of atoms - the smallest particles from which all substances are composed - contain a significant supply. It is this energy that is released in the form of radiation during the decay of radioactive elements. Radiation is dangerous to life, but nuclear reactions can be used to produce. Radiation is also used in medicine.
Radioactivity
Radioactivity is the property of the nuclei of unstable atoms to emit energy. Most heavy atoms are unstable, but lighter atoms have radioisotopes, i.e. radioactive isotopes. The reason for radioactivity is that atoms tend to become stable (see article " "). There are three types of radioactive radiation: alpha rays, beta rays And gamma rays. They are named after the first three letters of the Greek alphabet. Initially, the nucleus emits alpha or beta rays, and if it is still unstable, the nucleus emits gamma rays as well.  In the picture you see three atomic nuclei. They are unstable, and each of them emits one of three types of rays. Beta particles are electrons with very high energy. They arise from the decay of a neutron. Alpha particles consist of two protons and two neutrons. The nucleus of a helium atom has exactly the same composition. Gamma rays are high-energy electromagnetic radiation that travels at the speed of light.
In the picture you see three atomic nuclei. They are unstable, and each of them emits one of three types of rays. Beta particles are electrons with very high energy. They arise from the decay of a neutron. Alpha particles consist of two protons and two neutrons. The nucleus of a helium atom has exactly the same composition. Gamma rays are high-energy electromagnetic radiation that travels at the speed of light.
Alpha particles move slowly, and a layer of matter thicker than a sheet of paper traps them. They are no different from the nuclei of helium atoms. Scientists believe that helium on Earth is a product of natural radioactivity. An alpha particle flies less than 10 cm, and a sheet of thick paper will stop it. A beta particle flies about 1 meter in the air. A sheet of copper 1 millimeter thick can hold it back. The intensity of gamma rays drops by half when passing through a layer of lead of 13 millimeters or a layer of 120 meters.
Radioactive substances are transported in thick-walled lead containers to prevent radiation leakage. Exposure to radiation causes burns, cataracts, and cancer in humans. Radiation levels are measured using Geiger counter. This device makes a clicking noise when it detects radioactive radiation. Having emitted particles, the nucleus acquires a new atomic number and turns into the nucleus of another element. This process is called radioactive decay. If the new element is also unstable, the decay process continues until a stable nucleus is formed. For example, when a plutonium-2 atom (its mass is 242) emits an alpha particle whose relative atomic mass is 4 (2 protons and 2 neutrons), it turns into a uranium atom - 238 (atomic mass 238). Half life- this is the time during which half of all atoms in a sample of a given substance decay. Different ones have different half-lives. The half-life of radium-221 is 30 seconds, while that of uranium is 4.5 billion years.
Nuclear reactions
There are two types of nuclear reactions: nuclear fusion And fission (splitting) of the nucleus. "Synthesis" means "combination"; In nuclear fusion, two nuclei are combined and one is large. Nuclear fusion can only occur at very high temperatures. Fusion releases a huge amount of energy. In nuclear fusion, two nuclei are combined into one large one. In 1992, the COBE satellite discovered a special type of radiation in space, which confirms the theory that it was formed as a result of the so-called big bang. From the term fission it is clear that nuclei split apart, releasing nuclear energy. This is possible when nuclei are bombarded with neutrons and occurs in radioactive substances or in a special device called particle accelerator. The nucleus divides, emitting neutrons and releasing colossal energy.
 Nuclear power
Nuclear power
The energy released from nuclear reactions can be used to produce electricity and as a power source in nuclear submarines and aircraft carriers. The operation of a nuclear power plant is based on nuclear fission in nuclear reactors. A rod made of a radioactive substance such as uranium is bombarded with neutrons. Uranium nuclei split, emitting energy. This releases new neutrons. This process is called chain reaction. The power plant produces more energy per unit mass of fuel than any other power plant, but safety precautions and disposal of radioactive waste are extremely expensive.
 Nuclear weapon
Nuclear weapon
The action of nuclear weapons is based on the fact that the uncontrolled release of a huge amount of nuclear energy leads to a terrible explosion. At the end of World War II, the United States dropped atomic bombs on the Japanese cities of Hiroshima and Nagasaki. Hundreds of thousands of people died. Atomic bombs are based on fission reactions, hydrogen - on synthesis reactions. The picture shows the atomic bomb dropped on Hiroshima.
Radiocarbon method
The radiocarbon method determines the time that has passed since the death of an organism. Living things contain small amounts of carbon-14, a radioactive isotope of carbon. Its half-life is 5,700 years. When an organism dies, carbon-14 reserves in tissues are depleted, the isotope decays, and the remaining amount can be used to determine how long ago the organism died. Thanks to the radiocarbon dating method, you can find out how long ago the eruption occurred. To do this, they use insects and pollen frozen in lava.
How else is radioactivity used?
In industry, radiation is used to determine the thickness of a sheet of paper or plastic (see article ““). By the intensity of beta rays passing through the sheet, even slight heterogeneity in its thickness can be detected. Food products - fruits, meat - are irradiated with gamma rays to keep them fresh. Using radioactivity, doctors trace the path of a substance in the body. For example, to determine how sugar is distributed in a patient's body, a doctor might inject some carbon-14 into the sugar molecules and monitor the emission of the substance as it enters the body. Radiotherapy, that is, irradiating a patient with strictly dosed portions of radiation, kills cancer cells - overgrown cells of the body.
The energy contained in atomic nuclei and released during nuclear reactions and radioactive decay.
According to forecasts, organic fuels will be enough to meet humanity's energy needs for 4-5 decades. In the future, solar energy may become the main energy resource. The transition period requires a source of energy that is practically inexhaustible, cheap, renewable and does not pollute the environment. And although nuclear energy does not fully meet all of the above requirements, it is developing rapidly and our hope for solving the global energy crisis is connected with it.
The release of the internal energy of atomic nuclei is possible by the fission of heavy nuclei or the fusion of light nuclei.
Characteristics of the atom. An atom of any chemical element consists of a nucleus and electrons rotating around it. The nucleus of an atom consists of neutrons and protons. The term used as a common name for proton and neutron nucleon. Neutrons have no electrical charge, protons are positively charged, electrons - negative. The charge of a proton is equal in absolute value to the charge of an electron.
The number of protons of the Z nucleus coincides with its atomic number in the periodic table of Mendeleev. The number of neutrons in a nucleus, with few exceptions, is greater than or equal to the number of protons.
The mass of an atom is concentrated in the nucleus and is determined by the mass of nucleons. The mass of one proton is equal to the mass of one neutron. The mass of an electron is 1/1836 of the mass of a proton.
The dimension of atomic mass is used atomic mass unit(a.u.m), equal to 1.66·10 -27 kg. 1 amu approximately equal to the mass of one proton. The characteristic of an atom is the mass number A, equal to the total number of protons and neutrons.
The presence of neutrons allows two atoms to have different masses with the same electrical charges on the nucleus. The chemical properties of these two atoms will be the same; such atoms are called isotopes. In the literature, to the left of the element designation, the mass number is written at the top, and the number of protons at the bottom.
The nuclear fuel used in such reactors is isotope of uranium with atomic mass 235. Natural uranium is a mixture of three isotopes: uranium-234 (0.006%), uranium-235 (0.711%) and uranium-238 (99.283%). The uranium-235 isotope has unique properties - as a result of the absorption of a low-energy neutron, a uranium-236 nucleus is obtained, which is then split - divided into two approximately equal parts, called fission products (fragments). The nucleons of the original nucleus are distributed between the fission fragments, but not all of them - on average, 2-3 neutrons are released. As a result of fission, the mass of the original nucleus is not completely preserved; part of it is converted into energy, mainly into the kinetic energy of fission products and neutrons. The value of this energy for one atom of uranium 235 is about 200 MeV.
The core of a conventional 1000 MW reactor contains about 1 thousand tons of uranium, of which only 3 - 4% is uranium-235. Every day 3 kg of this isotope is consumed in the reactor. Thus, to supply the reactor with fuel, 430 kg of uranium concentrate must be processed daily, and this is an average of 2150 tons of uranium ore
As a result of the fission reaction, fast neutrons are produced in nuclear fuel. If they interact with neighboring nuclei of a fissile substance and, in turn, cause a fission reaction in them, an avalanche-like increase in the number of fission events occurs. This fission reaction is called a nuclear fission chain reaction.
Neutrons with energies less than 0.1 keV are most effective for the development of a fission chain reaction. They are called thermal because their energy is comparable to the average energy of thermal motion of molecules. For comparison, the energy possessed by neutrons produced during the decay of nuclei is 5 MeV. They are called fast neutrons. To use such neutrons in a chain reaction, their energy must be reduced (slowed down). These functions are performed by the moderator. In moderator substances, fast neutrons are scattered on nuclei, and their energy is converted into the energy of thermal motion of the atoms of the moderator substance. The most widely used moderators are graphite and liquid metals (primary circuit coolant).
The rapid development of a chain reaction is accompanied by the release of a large amount of heat and overheating of the reactor. To maintain a steady-state reactor mode, control rods made of materials that strongly absorb thermal neutrons, for example, boron or cadmium, are introduced into the reactor core.
The kinetic energy of decomposition products is converted into heat. Heat is absorbed by the coolant circulating in the nuclear reactor and transferred to the heat exchanger (1st closed circuit), where steam is produced (2nd circuit), which rotates the turbine of the turbogenerator. The coolant in the reactor is liquid sodium (1st circuit) and water (2nd circuit).
Uranium-235 is a non-renewable resource and if used entirely in nuclear reactors, it will disappear forever. Therefore, it is attractive to use the isotope uranium-238, which is found in much larger quantities, as the initial fuel. This isotope does not support a chain reaction under the influence of neutrons. But it can absorb fast neutrons, thereby forming uranium-239. In the nuclei of uranium-239, beta decay begins and neptunium-239 (not found in nature) is formed. This isotope also decays and becomes plutonium-239 (not found in nature). Plutonium-239 is even more susceptible to thermal neutron fission reactions. As a result of the fission reaction in the nuclear fuel plutonium-239, fast neutrons are formed, which, together with uranium, form new fuel and fission products that release heat in fuel elements (fuel elements). As a result, 20-30 times more energy can be obtained from a kilogram of natural uranium than in conventional nuclear reactors using uranium-235.
Modern designs use liquid sodium as a coolant. In this case, the reactor can operate at higher temperatures, thereby increasing the thermal efficiency of the power plant up to 40% .
However, the physical properties of plutonium: toxicity, low critical mass for spontaneous fission reactions, ignition in oxygen, brittleness and self-heating in the metallic state make it difficult to produce, process and handle. Therefore, breeder reactors are still less common than thermal neutron reactors.
For peaceful purposes, atomic energy is used in nuclear power plants. The share of nuclear power plants in global electricity production is about 14% .
As an example, consider the principle of generating electricity at the Voronezh NPP. A liquid metal coolant with an inlet temperature of 571 K is sent through channels through channels under a pressure of 157 ATM (15.7 MPa), which is heated in the reactor to 595 K. The metal coolant is sent to a steam generator, which receives cold water, which turns into steam with a pressure of 65.3 ATM (6.53 MPa). Steam is supplied to the blades of a steam turbine, which rotates a turbogenerator.
In nuclear reactors, the temperature of the steam produced is significantly lower than in the steam generator of thermal power plants using organic fuel. As a result, the thermal efficiency of nuclear power plants operating with water as a coolant is only 30%. For comparison, for power plants running on coal, oil or gas it reaches 40%.
Nuclear power plants are used in electricity and heat supply systems for the population, and mini-nuclear power plants on sea vessels (nuclear-powered ships, nuclear submarines) for electric drive of propellers).
For military purposes, nuclear energy is used in atomic bombs. The atomic bomb is a special fast neutron reactor , in which a fast uncontrolled chain reaction occurs with a high neutron multiplication factor. The nuclear reactor of an atomic bomb does not contain moderators. As a result, the dimensions and weight of the device become small.
The nuclear charge of a uranium-235 bomb is divided into two parts, in each of which a chain reaction is impossible. To create an explosion, one half of the charge is fired into the other, and when they are connected, an explosive chain reaction occurs almost instantly. An explosive nuclear reaction results in the release of enormous energy. In this case, a temperature of about one hundred million degrees is reached. A colossal increase in pressure occurs and a powerful blast wave is formed.
The first nuclear reactor was launched at the University of Chicago (USA) on December 2, 1942. The first atomic bomb was detonated on July 16, 1945 in New Mexico (Alamogordo). It was a device created on the principle of plutonium fission. The bomb consisted of plutonium surrounded by two layers of chemical explosive with fuses.
The first nuclear power plant to produce current in 1951 was the EBR-1 nuclear power plant (USA). In the former USSR - Obninsk Nuclear Power Plant (Kaluga region, gave power on June 27, 1954). The first nuclear power plant in the USSR with a fast neutron reactor with a capacity of 12 MW was launched in 1969 in the city of Dimitrovgrad. In 1984, there were 317 nuclear power plants operating in the world with a total capacity of 191 thousand MW, which amounted to 12% (1012 kWh) of global electricity production at that time. The world's largest nuclear power plant as of 1981 was the Biblis NPP (Germany), the thermal power of its reactors was 7800 MW.
Thermonuclear reactions are called nuclear reactions of fusion of light nuclei into heavier ones. The element used in nuclear fusion is hydrogen. The main advantage of thermonuclear synetz is the practically unlimited resources of feedstock, which can be extracted from sea water. Hydrogen in one form or another makes up 90% of all matter. The fuel for thermonuclear fusion contained in the world's oceans will last for more than 1 billion years (solar radiation and humanity in the solar system will not last much longer). The raw materials for thermonuclear fusion contained in 33 km of ocean water are equivalent in energy content to all solid fuel resources (there is 40 million times more water on Earth). The energy of deuterium contained in a glass of water is equivalent to burning 300 liters of gasoline.
There are 3 isotopes of hydrogen : their atomic masses are -1.2 (deuterium), 3 (tritium). These isotopes can reproduce nuclear reactions in which the total mass of the final reaction products is less than the total mass of the substances that entered into the reaction. The difference in mass, as in the case of a fission reaction, accounts for the kinetic energy of the reaction products. On average, the decrease in the mass of the substance involved in the thermonuclear fusion reaction is 1 amu. corresponds to the release of 931 MeV of energy:
H 2 + H 2 = H 3 + neutron +3.2 MeV,
H 2 + H 2 = H 3 + proton +4.0 MeV,
H 2 + H 3 = He 4 + neutron +17.6 MeV.
There is practically no tritium in nature. It can be obtained by the interaction of neutrons with lithium isotopes:
Li 6 + neutron = He 4 + H 3 + 4.8 MeV.
The fusion of nuclei of light elements does not occur naturally (excluding processes in space). In order to force nuclei to enter into a fusion reaction, high temperatures are required (about 107 -109 K). In this case, the gas is an ionized plasma. The problem of confining this plasma represents the main obstacle to the use of this method of energy production. Temperatures of about 10 million degrees are typical for the central part of the Sun. It is thermonuclear reactions that are the source of energy that provides radiation from the Sun and stars.
Currently, theoretical and experimental work is underway to study methods of magnetic and inertial plasma confinement.
Method of using magnetic fields. A magnetic field is created that penetrates the channel of moving plasma. The charged particles that make up the plasma, while moving in a magnetic field, are exposed to forces directed perpendicular to the movement of the particles and the magnetic field lines. Due to the action of these forces, the particles will move in a spiral along the field lines. The stronger the magnetic field, the denser the plasma flow becomes, thereby isolating itself from the walls of the shell.
Inertial plasma confinement. The reactor carries out thermonuclear explosions with a frequency of 20 explosions per second. To implement this idea, a particle of thermonuclear fuel is heated using focused radiation from 10 lasers to the ignition temperature of the fusion reaction in the time before it has time to scatter over a noticeable distance due to the thermal motion of atoms (10-9 s).
Thermonuclear fusion is the basis of the hydrogen (thermonuclear) bomb. In such a bomb, a self-sustaining thermonuclear reaction of an explosive nature occurs. The explosive is a mixture of deuterium and tritium. The energy of a nuclear fission bomb is used as a source of activation energy (a source of high temperatures). The world's first thermonuclear bomb was created in the USSR in 1953.
At the end of the 50s, the USSR began working on the idea of thermonuclear fusion in reactors of the TOKAMAK type (toroidal chamber in the magnetic field of a coil). The principle of operation is as follows: the toroidal chamber is evacuated and filled with a gas mixture of deuterium and tritium. A current of several million amperes is passed through the mixture. In 1-2 seconds, the temperature of the mixture rises to hundreds of thousands of degrees. Plasma is formed in the chamber. Further heating is carried out by injection of neutral deuterium and tritium atoms with an energy of 100 - 200 keV. The plasma temperature rises to tens of millions of degrees and a self-sustaining fusion reaction begins. After 10-20 minutes, heavy elements from the partially evaporating material of the chamber walls will accumulate in the plasma. The plasma cools down and thermonuclear combustion stops. The chamber must be turned off again and cleaned of accumulated impurities. The torus dimensions for a reactor thermal power of 5000 MW are as follows: Outer radius -10m; internal radius - 2.5 m.
Research to find a way to control thermonuclear reactions, i.e. The use of thermonuclear energy for peaceful purposes is developing with great intensity.
In 1991, at a joint European facility in the UK, significant energy release was achieved for the first time during controlled thermonuclear fusion. The optimal mode was maintained for 2 seconds and was accompanied by the release of energy of about 1.7 MW. The maximum temperature was 400 million degrees.
Thermonuclear electric generator. When deuterium is used as fusion fuel, two-thirds of the energy must be released in the form of kinetic energy of charged particles. Using electromagnetic methods, this energy can be converted into electrical energy.
Electricity can be obtained in stationary and pulsed operating modes of the installation. In the first case, the ions and electrons resulting from a self-sustaining fusion reaction are inhibited by a magnetic field. The ion current is separated from the electron current using a transverse magnetic field. The efficiency of such a system during direct braking will be about 50%, and the rest of the energy will turn into heat.
Fusion engines (not implemented). Scope of application: spacecraft. The fully ionized deuterium plasma at a temperature of 1 billion degrees Celsius is held in the form of a cord by the linear magnetic field of coils of superconductors. The working fluid is fed into the chamber through the walls, cooling them, and heated by flowing around the plasma cord. The axial velocity of ion outflow at the exit from the magnetic nozzle is 10,000 km/s.
In 1972, at one meeting of the Club of Rome - an organization studying the causes and searching for solutions to problems on a planetary scale - a report was made by scientists E. von Weinzsäcker, A. H. Lovins and produced the effect of an exploding bomb. According to the data given in the report, the planet's energy sources - coal, gas, oil and uranium - will be sufficient until 2030. To mine coal, from which you can get $1 worth of energy, you will need to expend energy costing 99 cents.
Uranium-235, which serves as fuel for nuclear power plants, is not so abundant in nature: only 5% of the total amount of uranium in the world, 2% of which is in Russia. Therefore, nuclear power plants can only be used for auxiliary purposes. The research of scientists who tried to obtain energy from plasma on TOKAMAKs remains an expensive exercise to this day. In 2000, reports emerged that the European Atomic Community (CERN) and Japan were building the first segment of TOKAMAK.
The salvation may not be the “peaceful atom” of a nuclear power plant, but the “military” one – the energy of a thermonuclear bomb.
Russian scientists called their invention an explosive combustion boiler (ECC). The operating principle of the PIC is based on the explosion of an ultra-small thermonuclear bomb in a special sarcophagus - a boiler. Explosions occur regularly. It is interesting that in a VBC the pressure on the walls of the boiler during an explosion is less than in the cylinders of an ordinary car.
For safe operation of the boiler, the internal diameter of the boiler must be at least 100 meters. Double steel walls and a 30-meter thick reinforced concrete shell will dampen vibrations. Only high-quality steel will be used to construct it, like two modern military battleships. It is planned to build the KVS for 5 years. In 2000, in one of the closed cities of Russia, a project was prepared for the construction of an experimental installation for a “bomb” of 2-4 kilotons of nuclear equivalent. The cost of this FAC is $500 million. Scientists have calculated that it will pay for itself in a year, and for another 50 years it will provide practically free electricity and heat. According to the project manager, the cost of energy equivalent to burning a ton of oil will be less than $10.
40 KVGs are capable of meeting the needs of the entire national energy sector. One hundred - all countries of the Eurasian continent.
In 1932, a positron was experimentally discovered - a particle with the mass of an electron, but with a positive charge. Soon it was suggested that charge symmetry exists in nature: a) every particle must have an antiparticle; b) the laws of nature do not change when all particles are replaced by corresponding antiparticles and vice versa. The antiproton and antineutron were discovered in the mid-50s. In principle, there can be antimatter consisting of atoms, the nuclei of which include antiprotons and antineutrons, and their shell is formed by positrons.
Clots of antimatter of cosmological sizes would constitute antiworlds, but they are not found in nature. Antimatter is synthesized only on a laboratory scale. Thus, in 1969, at the Serpukhov accelerator, Soviet physicists detected antihelium nuclei consisting of two antiprotons and one antineutron.
With regard to the possibilities of energy conversion, antimatter is notable for the fact that when it comes into contact with matter, annihilation (destruction) occurs with the release of colossal energy (both types of matter disappear, turning into radiation). Thus, an electron and a positron, annihilating, generate two photons. One type of matter—charged massive particles—transforms into another type of matter—neutral massless particles. Using Einstein's relation about the equivalence of energy and mass (E=mc 2), it is not difficult to calculate that the annihilation of one gram of matter produces the same energy that can be obtained by burning 10,000 tons of coal, and one ton of antimatter would be enough to provide energy for the entire planet for a year.
Astrophysicists believe that it is annihilation that provides the gigantic energy of quasi-stellar objects - quasars.
In 1979, a group of American physicists managed to register the presence of natural antiprotons. They were brought by cosmic rays.
The use of nuclear energy in the modern world turns out to be so important that if we woke up tomorrow and the energy from the nuclear reaction had disappeared, the world as we know it would probably cease to exist. Peace forms the basis of industrial production and life in countries such as France and Japan, Germany and Great Britain, the USA and Russia. And if the last two countries are still able to replace nuclear energy sources with thermal stations, then for France or Japan this is simply impossible.
The use of nuclear energy creates many problems. Basically, all these problems are related to the fact that using the binding energy of the atomic nucleus (which we call nuclear energy) for one’s benefit, a person receives a significant evil in the form of highly radioactive waste that cannot simply be thrown away. Waste from nuclear energy sources must be processed, transported, buried, and stored for a long time in safe conditions.
Pros and cons, benefits and harms of using nuclear energy
Let's consider the pros and cons of using atomic-nuclear energy, their benefits, harm and significance in the life of Mankind. It is obvious that nuclear energy today is needed only by industrialized countries. That is, peaceful nuclear energy is mainly used in facilities such as factories, processing plants, etc. It is energy-intensive industries that are remote from sources of cheap electricity (such as hydroelectric power plants) that use nuclear power plants to ensure and develop their internal processes.
Agrarian regions and cities do not have much need for nuclear energy. It is quite possible to replace it with thermal and other stations. It turns out that the mastery, acquisition, development, production and use of nuclear energy is for the most part aimed at meeting our needs for industrial products. Let's see what kind of industries they are: automotive industry, military production, metallurgy, chemical industry, oil and gas complex, etc.
Does a modern person want to drive a new car? Want to dress in fashionable synthetics, eat synthetics and pack everything in synthetics? Want colorful products in different shapes and sizes? Wants all new phones, TVs, computers? Do you want to buy a lot and often change the equipment around you? Do you want to eat delicious chemical food from colored packages? Do you want to live in peace? Want to hear sweet speeches from the TV screen? Does he want there to be a lot of tanks, as well as missiles and cruisers, as well as shells and guns?
And he gets it all. It does not matter that in the end the discrepancy between word and deed leads to war. It doesn't matter that recycling it also requires energy. For now the man is calm. He eats, drinks, goes to work, sells and buys.
And all this requires energy. And this also requires a lot of oil, gas, metal, etc. And all these industrial processes require nuclear energy. Therefore, no matter what anyone says, until the first industrial thermonuclear fusion reactor is put into production, nuclear energy will only develop.
We can safely list everything that we are used to as the advantages of nuclear energy. The downside is the sad prospect of imminent death due to the collapse of resource depletion, problems of nuclear waste, population growth and degradation of arable land. In other words, nuclear energy allowed man to begin to take control of nature even more, raping it beyond measure to such an extent that in a few decades he overcame the threshold of reproduction of basic resources, launching the process of collapse of consumption between 2000 and 2010. This process objectively no longer depends on the person.
Everyone will have to eat less, live less and enjoy the natural environment less. Here lies another plus or minus of nuclear energy, which is that countries that have mastered the atom will be able to more effectively redistribute the scarce resources of those who have not mastered the atom. Moreover, only the development of the thermonuclear fusion program will allow humanity to simply survive. Now let’s explain in detail what kind of “beast” this is - atomic (nuclear) energy and what it is eaten with.
Mass, matter and atomic (nuclear) energy
We often hear the statement that “mass and energy are the same thing,” or such judgments that the expression E = mc2 explains the explosion of an atomic (nuclear) bomb. Now that you have a first understanding of nuclear energy and its applications, it would be truly unwise to confuse you with statements such as “mass equals energy.” In any case, this way of interpreting the great discovery is not the best. Apparently, this is just the wit of young reformists, “Galileans of the new time.” In fact, the prediction of the theory, which has been verified by many experiments, only says that energy has mass.
We will now explain the modern point of view and give a short overview of the history of its development.
When the energy of any material body increases, its mass increases, and we attribute this additional mass to the increase in energy. For example, when radiation is absorbed, the absorber becomes hotter and its mass increases. However, the increase is so small that it remains beyond the accuracy of measurements in ordinary experiments. On the contrary, if a substance emits radiation, then it loses a drop of its mass, which is carried away by the radiation. A broader question arises: is not the entire mass of matter determined by energy, i.e., is there not a huge reserve of energy contained in all matter? Many years ago, radioactive transformations responded positively to this. When a radioactive atom decays, a huge amount of energy is released (mostly in the form of kinetic energy), and a small part of the atom's mass disappears. The measurements clearly show this. Thus, energy carries away mass with it, thereby reducing the mass of matter.
Consequently, part of the mass of matter is interchangeable with the mass of radiation, kinetic energy, etc. That is why we say: “energy and matter are partially capable of mutual transformations.” Moreover, we can now create particles of matter that have mass and are capable of being completely converted into radiation, which also has mass. The energy of this radiation can transform into other forms, transferring its mass to them. Conversely, radiation can turn into particles of matter. So instead of “energy has mass,” we can say “particles of matter and radiation are interconvertible, and therefore capable of interconversion with other forms of energy.” This is the creation and destruction of matter. Such destructive events cannot occur in the realm of ordinary physics, chemistry and technology, they must be sought either in the microscopic but active processes studied by nuclear physics, or in the high-temperature crucible of atomic bombs, in the Sun and stars. However, it would be unreasonable to say that "energy is mass." We say: “energy, like matter, has mass.”
Mass of ordinary matter
We say that the mass of ordinary matter contains within itself a huge supply of internal energy, equal to the product of mass by (the speed of light)2. But this energy is contained in the mass and cannot be released without the disappearance of at least part of it. How did such an amazing idea come about and why was it not discovered earlier? It had been proposed before - experiment and theory in different forms - but until the twentieth century the change in energy was not observed, because in ordinary experiments it corresponds to an incredibly small change in mass. However, we are now confident that a flying bullet, due to its kinetic energy, has additional mass. Even at a speed of 5000 m/sec, a bullet that weighed exactly 1 g at rest will have a total mass of 1.00000000001 g. White-hot platinum weighing 1 kg will only add 0.000000000004 kg and practically no weighing will be able to register these changes. It is only when enormous reserves of energy are released from the atomic nucleus, or when atomic "projectiles" are accelerated to speeds close to the speed of light, that the mass of energy becomes noticeable.
On the other hand, even a subtle difference in mass marks the possibility of releasing a huge amount of energy. Thus, hydrogen and helium atoms have relative masses of 1.008 and 4.004. If four hydrogen nuclei could combine into one helium nucleus, the mass of 4.032 would change to 4.004. The difference is small, only 0.028, or 0.7%. But it would mean a gigantic release of energy (mainly in the form of radiation). 4.032 kg of hydrogen would produce 0.028 kg of radiation, which would have an energy of about 600000000000 Cal.
Compare this to the 140,000 Cals released when the same amount of hydrogen combines with oxygen in a chemical explosion.
Ordinary kinetic energy makes a significant contribution to the mass of very fast protons produced in cyclotrons, and this creates difficulties when working with such machines.
Why do we still believe that E=mc2
Now we perceive this as a direct consequence of the theory of relativity, but the first suspicions arose towards the end of the 19th century, in connection with the properties of radiation. It seemed likely then that the radiation had mass. And since radiation carries, as if on wings, at a speed with energy, or rather, it itself is energy, an example of mass has appeared that belongs to something “immaterial”. The experimental laws of electromagnetism predicted that electromagnetic waves should have "mass." But before the creation of the theory of relativity, only unbridled imagination could extend the ratio m=E/c2 to other forms of energy.
All types of electromagnetic radiation (radio waves, infrared, visible and ultraviolet light, etc.) share some common features: they all propagate in vacuum at the same speed and all transfer energy and momentum. We imagine light and other radiation in the form of waves propagating at a high but certain speed c = 3*108 m/sec. When light strikes an absorbing surface, heat is generated, indicating that the stream of light carries energy. This energy must propagate along with the flow at the same speed of light. In fact, the speed of light is measured exactly this way: by the time it takes a portion of light energy to travel a long distance.
When light hits the surface of some metals, it knocks out electrons that fly out just as if they had been hit by a compact ball. , apparently, is distributed in concentrated portions, which we call “quanta”. This is the quantum nature of the radiation, despite the fact that these portions are apparently created by waves. Each piece of light with the same wavelength has the same energy, a certain “quantum” of energy. Such portions rush at the speed of light (in fact, they are light), transferring energy and momentum (momentum). All this makes it possible to attribute a certain mass to the radiation - a certain mass is assigned to each portion.
When light is reflected from a mirror, no heat is released, because the reflected beam carries away all the energy, but the mirror is subject to pressure similar to the pressure of elastic balls or molecules. If, instead of a mirror, the light hits a black absorbing surface, the pressure becomes half as much. This indicates that the beam carries the amount of motion rotated by the mirror. Therefore, light behaves as if it had mass. But is there any other way to know that something has mass? Does mass exist in its own right, such as length, green color, or water? Or is it an artificial concept defined by behavior like Modesty? Mass, in fact, is known to us in three manifestations:
- A. A vague statement characterizing the amount of “substance” (Mass from this point of view is inherent in matter - an entity that we can see, touch, push).
- B. Certain statements linking it with other physical quantities.
- B. Mass is conserved.
It remains to determine the mass in terms of momentum and energy. Then any moving thing with momentum and energy must have "mass". Its mass should be (momentum)/(velocity).
Theory of relativity
The desire to link together a series of experimental paradoxes concerning absolute space and time gave rise to the theory of relativity. Two kinds of experiments with light gave conflicting results, and experiments with electricity further aggravated this conflict. Then Einstein proposed changing the simple geometric rules for adding vectors. This change is the essence of his “special theory of relativity.”
For low speeds (from the slowest snail to the fastest of rockets), the new theory agrees with the old one.
At high speeds, comparable to the speed of light, our measurement of lengths or time is modified by the movement of the body relative to the observer, in particular, the mass of the body becomes greater the faster it moves.
Then the theory of relativity declared that this increase in mass was completely general. At normal speeds there is no change, and only at a speed of 100,000,000 km/h does the mass increase by 1%. However, for electrons and protons emitted from radioactive atoms or modern accelerators, it reaches 10, 100, 1000%…. Experiments with such high-energy particles provide excellent confirmation of the relationship between mass and velocity.
At the other edge there is radiation that has no rest mass. It is not a substance and cannot be kept at rest; it simply has mass and moves with speed c, so its energy is equal to mc2. We talk about quanta as photons when we want to note the behavior of light as a stream of particles. Each photon has a certain mass m, a certain energy E=mс2 and momentum (momentum).
Nuclear transformations
In some experiments with nuclei, the masses of atoms after violent explosions do not add up to the same total mass. The released energy carries with it some part of the mass; the missing piece of atomic material appears to have disappeared. However, if we assign the mass E/c2 to the measured energy, we find that the mass is conserved.
Annihilation of matter
We are accustomed to thinking of mass as an inevitable property of matter, so the transition of mass from matter to radiation - from a lamp to an escaping ray of light - looks almost like the destruction of matter. One more step - and we will be surprised to discover what is actually happening: positive and negative electrons, particles of matter, joining together, are completely converted into radiation. The mass of their matter turns into an equal mass of radiation. This is a case of disappearance of matter in the most literal sense. As if in focus, in a flash of light.
Measurements show that (energy, radiation during annihilation)/ c2 is equal to the total mass of both electrons - positive and negative. An antiproton combines with a proton and annihilates, usually releasing lighter particles with high kinetic energy.
Creation of matter
Now that we have learned to manage high-energy radiation (ultra-short-wave X-rays), we can prepare particles of matter from the radiation. If a target is bombarded with such rays, they sometimes produce a pair of particles, for example positive and negative electrons. And if we again use the formula m=E/c2 for both radiation and kinetic energy, then the mass will be conserved.
Simply about the complex – Nuclear (Atomic) energy
- Gallery of images, pictures, photographs.
- Nuclear energy, atomic energy - fundamentals, opportunities, prospects, development.
- Interesting facts, useful information.
- Green news – Nuclear energy, atomic energy.
- Links to materials and sources – Nuclear (Atomic) energy.

The dependence of the binding energy per nucleon on the number of nucleons in the nucleus is shown in the graph.
The energy required to split a nucleus into individual nucleons is called binding energy. The binding energy per nucleon is not the same for different chemical elements and, even, isotopes of the same chemical element. The specific binding energy of a nucleon in a nucleus varies, on average, from 1 MeV for light nuclei (deuterium) to 8.6 MeV for medium-weight nuclei (A≈100). For heavy nuclei (A≈200), the specific binding energy of a nucleon is less than for nuclei of average weight, by approximately 1 MeV, so their transformation into nuclei of average weight (division into 2 parts) is accompanied by the release of energy in an amount of about 1 MeV per nucleon, or about 200 MeV per nucleus. The transformation of light nuclei into heavier nuclei gives an even greater energy gain per nucleon. For example, the reaction between deuterium and tritium
1 D²+ 1 T³→ 2 He 4 + 0 n 1
is accompanied by the release of energy 17.6 MeV, that is, 3.5 MeV per nucleon.
Release of nuclear energy
Exothermic nuclear reactions that release nuclear energy are known.
Typically, a nuclear fission chain reaction of uranium-235 or plutonium nuclei is used to produce nuclear energy. Nuclei fission when a neutron hits them, producing new neutrons and fission fragments. Fission neutrons and fission fragments have high kinetic energy. As a result of collisions of fragments with other atoms, this kinetic energy is quickly converted into heat.
Another way to release nuclear energy is nuclear fusion. In this case, two nuclei of light elements combine into one heavy one. Such processes occur on the Sun.
Many atomic nuclei are unstable. Over time, some of these nuclei spontaneously transform into other nuclei, releasing energy. This phenomenon is called radioactive decay.
Applications of nuclear energy
Fusion energy is used in a hydrogen bomb.
Notes
see also
Links
International agreements
- Convention on Early Notification of a Nuclear Accident (Vienna, 1986)
- Convention on the Physical Protection of Nuclear Material (Vienna, 1979)
- Vienna Convention on Civil Liability for Nuclear Damage
- Joint Convention on the Safety of Spent Fuel Management and the Safety of Radioactive Waste Management
Literature
- Clarfield, Gerald H. and William M. Wiecek (1984). Nuclear America: Military and Civilian Nuclear Power in the United States 1940-1980, Harper & Row.
- Cooke, Stephanie (2009). In Mortal Hands: A Cautionary History of the Nuclear Age, Black Inc.
- Cravens Gwyneth Power to Save the World: the Truth about Nuclear Energy. - New York: Knopf, 2007. - ISBN 0-307-26656-7
- Elliott, David (2007). Nuclear or Not? Does Nuclear Power Have a Place in a Sustainable Energy Future?, Palgrave.
- Falk, Jim (1982). Global Fission: The Battle Over Nuclear Power, Oxford University Press.
- Ferguson, Charles D., (2007). Nuclear Energy: Balancing Benefits and Risks Council on Foreign Relations.
- Herbst, Alan M. and George W. Hopley (2007). Nuclear Energy Now: Why the Time has come for the World’s Most Misunderstood Energy Source, Wiley.
- Schneider, Mycle, Steve Thomas, Antony Froggatt, Doug Koplow (August 2009). The World Nuclear Industry Status Report, German Federal Ministry of Environment, Nature Conservation and Reactor Safety.
- Walker, J. Samuel (1992). Containing the Atom: Nuclear Regulation in a Changing Environment, 1993-1971
- Walker, J. Samuel (2004). Three Mile Island: A Nuclear Crisis in Historical Perspective, Berkeley: University of California Press.
- Weart, Spencer R. The Rise of Nuclear Fear. Cambridge, MA: Harvard University Press, 2012. ISBN 0-674-05233-1
| Nuclear technology | |
|---|---|
| Engineering | |
| Materials | |
| Nuclear power | |
| Nuclear medicine | |
| Nuclear weapon | |
Wikimedia Foundation. 2010.
- Kossman, Bernhard
- Zimmerman, Albert Karl Heinrich
See what “Nuclear energy” is in other dictionaries:
NUCLEAR POWER- (atomic energy) internal energy of atomic nuclei released during nuclear transformations (nuclear reactions). nuclear binding energy. mass defect Nucleons (protons and neutrons) in the nucleus are firmly held by nuclear forces. To remove a nucleon from a nucleus,... ...
NUCLEAR POWER- (nuclear energy), internal energy at. nucleus, released during nuclear transformations. The energy that must be expended to split a nucleus into its constituent nucleons is called. nuclear binding energy?st. This is max. energy towards heaven can be released.… … Physical encyclopedia
NUCLEAR POWER- NUCLEAR ENERGY, ENERGY released during a nuclear reaction as a result of the transition of MASS into energy as described in the equation: E=mc2 (where E is energy, m is mass, c is the speed of light); it was derived by A. EINSTEIN in his THEORY OF RELATIVITY.... ... Scientific and technical encyclopedic dictionary
NUCLEAR POWER- (nuclear energy) see () () ... Big Polytechnic Encyclopedia
NUCLEAR POWER Modern encyclopedia
NUCLEAR POWER- (atmic energy) internal energy of atomic nuclei, released during certain nuclear transformations. The use of nuclear energy is based on the implementation of chain reactions of fission of heavy nuclei and thermonuclear fusion reactions of light nuclei... Big Encyclopedic Dictionary
Nuclear power- (atomic energy), the internal energy of atomic nuclei released during certain nuclear reactions. The use of nuclear energy is based on the implementation of chain reactions of fission of heavy nuclei and thermonuclear fusion reactions of light nuclei (see... ... Illustrated Encyclopedic Dictionary
Nuclear power- internal energy of the atomic nucleus associated with the movement and interaction of the nucleons (neutrons and protons) forming the nucleus. Released during radioactive decay or nuclear fission and fusion reactions. Rapid release of nuclear energy... ...Nautical Dictionary